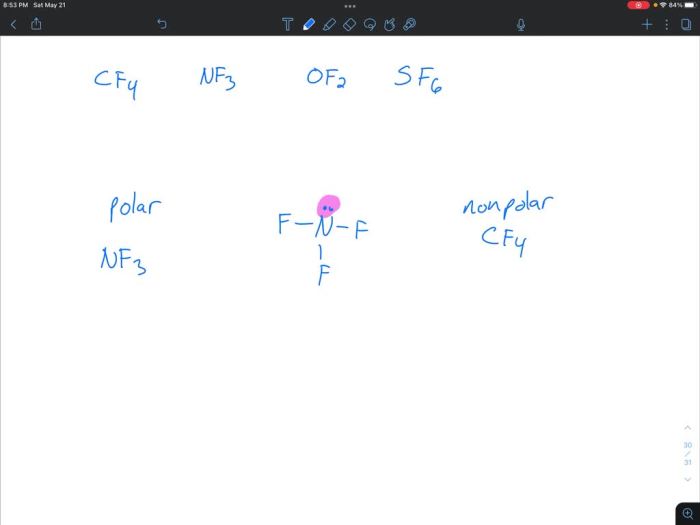
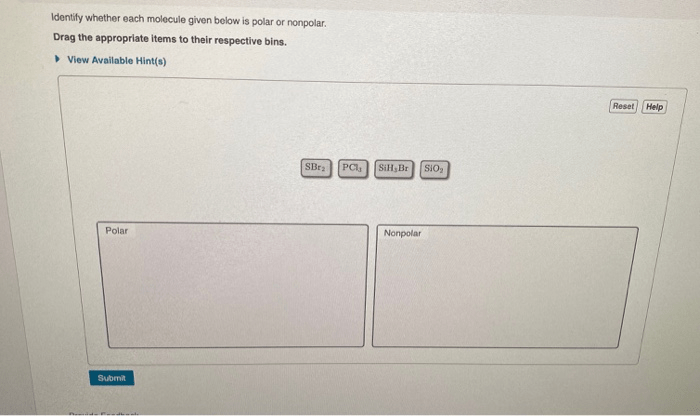
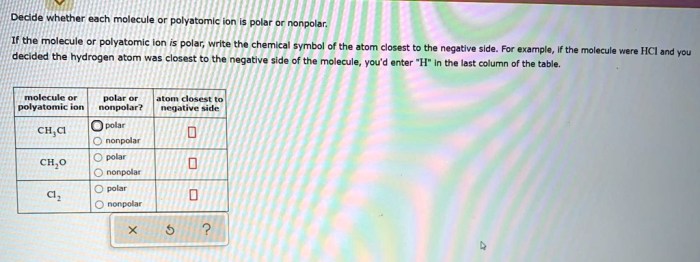
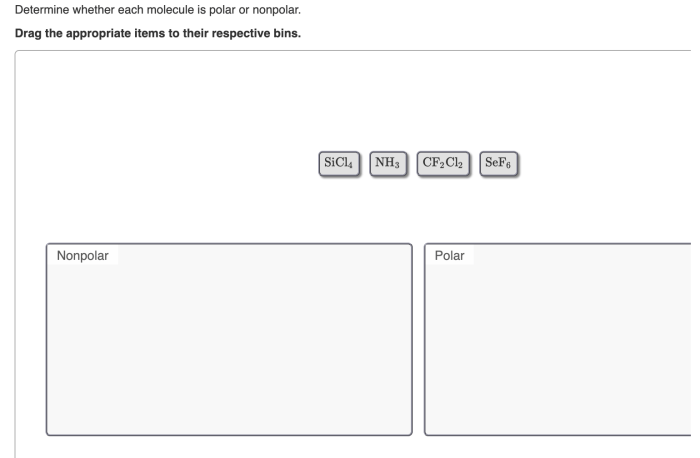
Determine whether each molecule given below is polar or nonpolar – Embarking on a journey into the fascinating realm of molecular polarity, we delve into the fundamental concepts that govern the behavior of molecules and their interactions with their surroundings. Polarity, a defining characteristic of molecules, plays a pivotal role in shaping their properties and influencing their participation in chemical processes.
In this exploration, we will unravel the intricacies of molecular polarity, delving into the factors that contribute to its existence and exploring the methods employed to determine the polarity of molecules. Through real-world examples and practical applications, we will gain a comprehensive understanding of this essential aspect of molecular science.
Concepts of Polarity
Polarity refers to the uneven distribution of electrons within a molecule, resulting in a separation of positive and negative charges. A molecule is considered polar if it has a net dipole moment, which is a measure of the strength and direction of the charge separation.
Nonpolar molecules, on the other hand, have an even distribution of electrons, resulting in no net dipole moment.
Factors that contribute to molecular polarity include electronegativity and molecular geometry. Electronegativity is a measure of an atom’s ability to attract electrons towards itself. The greater the difference in electronegativity between the atoms in a molecule, the more polar the molecule will be.
Molecular geometry also plays a role in polarity. Molecules with asymmetrical shapes, such as water, are more likely to be polar than molecules with symmetrical shapes, such as carbon dioxide.
Methods for Determining Polarity
There are several methods used to determine the polarity of molecules:
- Dipole moment measurements:This method involves measuring the net dipole moment of a molecule using techniques such as microwave spectroscopy or dielectric constant measurements.
- Electronegativity calculations:By calculating the difference in electronegativity between the atoms in a molecule, it is possible to predict the polarity of the molecule. The greater the difference in electronegativity, the more polar the molecule.
- Molecular geometry analysis:The geometry of a molecule can be used to determine its polarity. Molecules with asymmetrical shapes are more likely to be polar than molecules with symmetrical shapes.
Examples of Polar and Nonpolar Molecules
| Molecular Formula | Structure | Polarity |
|---|---|---|
| H2O |  |
Polar |
| CO2 |  |
Nonpolar |
| NH3 |  |
Polar |
| CH4 |  |
Nonpolar |
Applications of Polarity, Determine whether each molecule given below is polar or nonpolar
Molecular polarity has numerous applications in various fields:
- Solvent properties:Polar solvents, such as water, are able to dissolve polar solutes, while nonpolar solvents, such as hexane, are able to dissolve nonpolar solutes.
- Chemical reactions:Polarity plays a role in chemical reactions, such as acid-base reactions and redox reactions. Polar molecules are more reactive than nonpolar molecules.
- Biological processes:Polarity is essential for many biological processes, such as the transport of molecules across cell membranes and the interactions between proteins and other molecules.
Common Queries: Determine Whether Each Molecule Given Below Is Polar Or Nonpolar
What factors contribute to molecular polarity?
Molecular polarity arises from the uneven distribution of electrons within a molecule, resulting from differences in electronegativity between atoms and the geometry of the molecule.
How can we determine the polarity of a molecule?
Several methods are employed to determine molecular polarity, including dipole moment measurements, electronegativity calculations, and molecular geometry analysis.
What are the practical applications of molecular polarity?
Molecular polarity finds applications in diverse fields, including solvent properties, chemical reactions, and biological processes, influencing molecular interactions and shaping their behavior.