Unit periodic trends atomic size trend – ws #2 – Unit Periodic Trends: Atomic Size Trend – WS #2 delves into the fascinating realm of atomic size and its profound influence on the chemical properties of elements. This comprehensive guide unveils the intricate relationship between atomic size and the number of electron shells, providing a solid foundation for understanding the periodic trends that shape the behavior of matter.
As we traverse the periodic table, we uncover the factors that govern atomic size, including effective nuclear charge and shielding effects. These concepts lay the groundwork for comprehending the diverse atomic sizes observed across the elements. Moreover, we explore the practical applications of atomic size trends, demonstrating their significance in predicting chemical reactivity, materials science, and the burgeoning field of nanotechnology.
Unit Periodic Trends
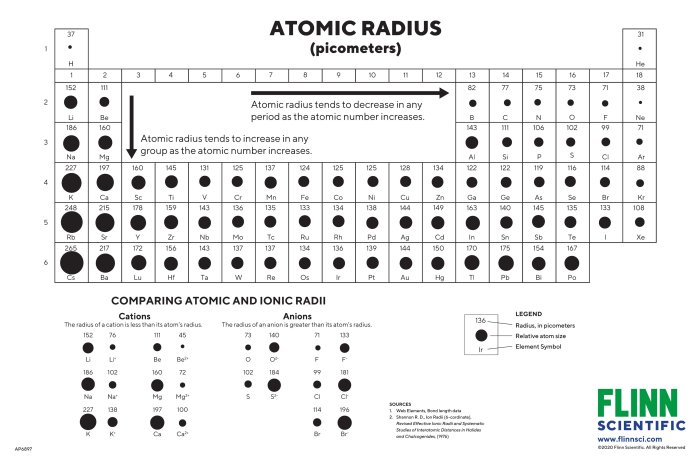
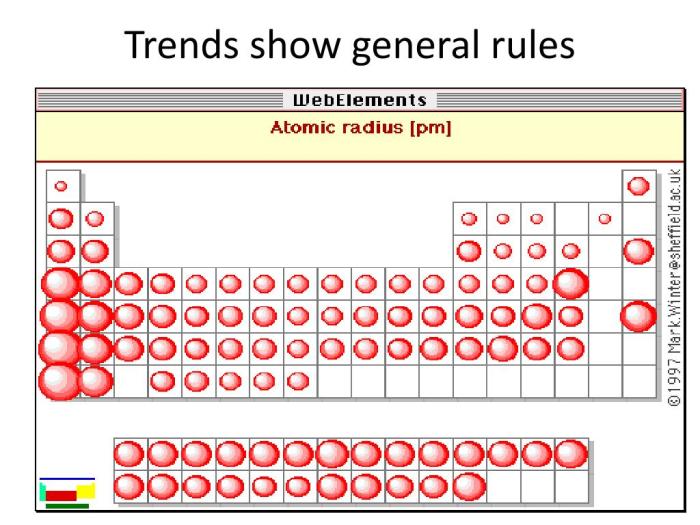
Periodic trends are patterns in the chemical and physical properties of elements that repeat periodically as the atomic number increases. One of the most important periodic trends is the trend in atomic size.
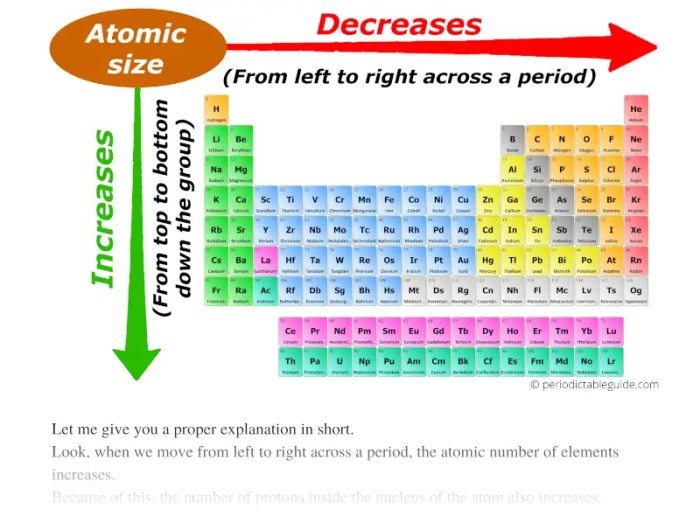
Atomic size is a measure of the distance from the nucleus to the outermost electron shell. It is typically measured in picometers (pm) or angstroms (Å). The atomic size of an element generally decreases across a period (from left to right) and increases down a group (from top to bottom) in the periodic table.
Relationship between Atomic Size and the Number of Electron Shells, Unit periodic trends atomic size trend – ws #2
The number of electron shells in an atom is related to its atomic number. The atomic number is the number of protons in the nucleus, and it determines the number of electrons in the atom. As the atomic number increases, the number of electron shells also increases.
The outermost electron shell is the valence shell. The valence electrons are the electrons that participate in chemical bonding. The size of the valence shell determines the atomic size of an element.
Examples of How Atomic Size Affects the Chemical Properties of Elements
- Reactivity:Smaller atoms are more reactive than larger atoms because they have a higher effective nuclear charge. The effective nuclear charge is the net positive charge experienced by the electrons in the valence shell. The higher the effective nuclear charge, the more strongly the electrons are attracted to the nucleus, and the more reactive the element.
- Melting point:Smaller atoms have higher melting points than larger atoms because they have stronger interatomic forces. Interatomic forces are the forces that hold atoms together in a solid. The stronger the interatomic forces, the higher the melting point.
- Boiling point:Smaller atoms have lower boiling points than larger atoms because they have weaker intermolecular forces. Intermolecular forces are the forces that hold molecules together in a liquid. The weaker the intermolecular forces, the lower the boiling point.
Q&A: Unit Periodic Trends Atomic Size Trend – Ws #2
What is the periodic trend of atomic size?
The periodic trend of atomic size refers to the general decrease in atomic size across a period (row) of the periodic table from left to right and the increase in atomic size down a group (column).
How does atomic size affect the chemical properties of elements?
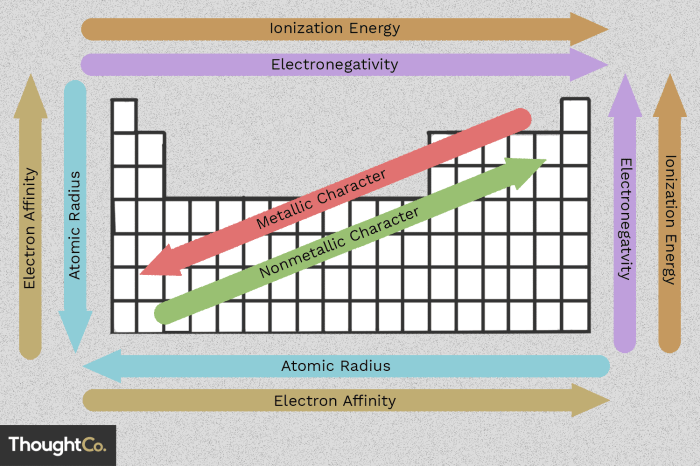
Atomic size influences various chemical properties, such as ionization energy, electronegativity, and reactivity. Smaller atoms have higher ionization energies and electronegativities, while larger atoms are more reactive.
What factors influence the size of an atom?
The size of an atom is primarily determined by the number of electron shells and the effective nuclear charge experienced by the outermost electrons.